Abstract

Background Patients with polycythemia vera (PV) are treated with periodic therapeutic phlebotomy (TP) alone or in combination with cytoreductive therapies to maintain hematocrit (HCT) levels below 45%, per NCCN and ELN guidelines. Since they are seen only periodically, patients with PV likely spend significant time with HCT levels above 45%, thereby increasing their risk of thrombosis [Marchioli NEJM 2013]. Indeed, a study of more than 4,000 US patients with PV in 2018 and 2019 found that only 22% maintained HCT<45% at all measurements and, of those with a prior thrombotic event, 36% had ≥1 additional thrombotic event within 5 years of treatment initiation, the most common being deep vein thrombosis (DVT) and stroke [Verstovsek, ASH 2020].
Excessive erythrocytosis in PV occurs despite iron deficiency, which is due to TP and utilization for red cell production. Hepcidin is also downregulated in PV. Patients report that fatigue is the most prevalent and severe systemic PV-associated symptom [Scherber, Cancer 2016]. This may be indicative of symptomatic iron deficiency, representing an unaddressed clinical challenge in the management of patients with PV.
In the phase 2 REVIVE study (NCT04057040), we demonstrated the clinical utility of rusfertide (PTG-300), a potent hepcidin mimetic being assessed as a treatment for uncontrolled PV-associated erythrocytosis. In REVIVE, rusfertide demonstrated good tolerability, as well as consistent and durable HCT control with improvements in iron deficiency in patients with excessive TP needs despite standard of care PV therapy [Hoffman, ASH 2021].
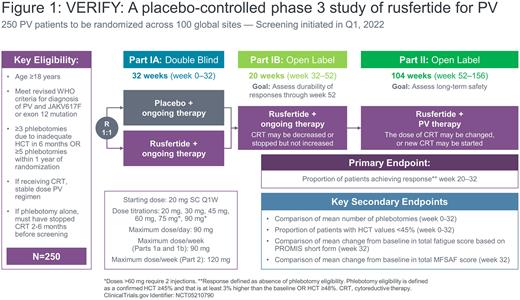
Study Design and Methods VERIFY (NCT05210790) is a phase 3, multicenter, global, randomized trial comparing the efficacy and safety of rusfertide versus placebo when added to ongoing therapy for PV (Figure 1). The study population comprises PV patients who require frequent TP, with or without concurrent cytoreductive therapy, to control their HCT.
The study is comprised of three parts:
Part 1a: 1:1 randomized, double-blind, placebo-controlled, add-on phase with parallel-groups lasting 32 weeks.
Part 1b: open-label treatment phase with cross-over for previous placebo-treated patients. During this phase, all patients who completed Part 1a successfully receive rusfertide for 20 weeks (week 32 to 52).
Part 2: Long term extension phase during which all patients who complete Part 1b will continue to receive rusfertide for 104 weeks (week 52 to 156).
Major inclusion criteria include: PV diagnosis (2016 WHO criteria) with either JAK2 V617F mutation or JAK2 exon 12 mutation; ≥3 TP in the previous 6 months or ≥5 TP in the previous 12 months due to inadequate HCT control; HCT <45%, white blood cells 4-20 x 109/L and platelets 100-1000 x 109/L at week 0 prior to randomization; a stable PV therapy regimen in patients receiving cytoreductive therapy at randomization; and cessation of cytoreductive therapy 2-6 months before screening in patients treated with TP alone.
Major exclusion criteria include: clinically significant thrombosis, or active or chronic bleeding deemed clinically significant by the investigator, within 2 months before randomization, and a history of invasive malignancy within the previous 5 years.
Patients who meet eligibility criteria are stratified by their ongoing PV therapy and randomized 1:1 to treatment with rusfertide or placebo in addition to the patient's ongoing PV therapy at week 0. The dose of cytoreductive therapy in Part 1a and Part 1b may be decreased for safety but may not be increased for efficacy, including control of HCT, thrombocytosis and/or leukocytosis.
The primary endpoint is the proportion of patients achieving a response in part 1A from week 20 through week 32. A response is defined as absence of TP eligibility. TP eligibility is defined as either: a confirmed hematocrit ≥45% and that is at least 3% higher than the baseline hematocrit OR a HCT ≥48%.
Secondary endpoints are: mean number of TPs from week 0 to 32; proportion of patients with HCT <45% from week 0 to 32; mean change from baseline to week 32 in total fatigue score as measured by the PROMIS Short Form, and mean change from baseline to week 32 in total symptom score as measured by the MFSAF.
The VERIFY study was initiated in January 2022. Sites are being opened globally with an aim of enrolling approximately 250 patients.
Disclosures
Verstovsek:CTI BioPharma Corp.: Research Funding; Gilead: Research Funding; Protagonist Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Genentech: Research Funding; NS Pharma: Research Funding; Novartis: Consultancy, Research Funding; Roche: Research Funding; PharmaEssentia: Research Funding; Promedior: Research Funding; ItalPharma: Research Funding; Incyte: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Hoffman:Ionis: Consultancy; Silence Therapeutics: Consultancy; Scholar Rock: Research Funding; Repare: Research Funding; Protagonist Therapeutics: Consultancy; Turning Point: Research Funding; Novartis: Other: Chair DSMB; Novartis: Research Funding; Abbvie: Other: Chair DSMB, Research Funding. Koschmieder:Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; CTI Biopharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; PharmaEssentia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Alexion: Other: Travel support; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Imago Biosciences: Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Karthos: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Sierra Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; RWTH Aachen University: Patents & Royalties: BET Inhibitor; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; AOP Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Geron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding. Passamonti:Abbvie: Honoraria; Janssen: Honoraria; BMS: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Protagonist Therapeutics: Membership on an entity's Board of Directors or advisory committees. Valone:Protagonist Therapeutics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Modi:Protagonist Therapeutics: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Khanna:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. O'Connor:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gupta:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kiladjian:Incyte: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal